Car batteries: types and characteristics
A car battery (rechargeable acid battery) is a seasonal thing, although it is used all year round. After all, it is easy to turn the crankshaft in the warm season. But the moment it gets cold, the full blown question of the starter’s “difficult fate” raises immediately. It is the one which tries to turn into a purely active resistance, consuming much current. As a result, the battery is trying to fail. If it is faulty, you won’t be able to start the car’s engine in normal mode. How to prevent the “dying” of the battery, how to choose good and workable one – this is discussed in the article.
What are batteries in cars for?
The purpose of the battery is to start the engine, as well as perform the functions of a power source in the car’s on-board network when the engine is turned off. The car battery also acts as a voltage regulator for the vehicle’s electrical system.
The most common are batteries with a nominal voltage of 12 volts. They can be found in cars, vans, light and medium trucks. Batteries with a voltage of 6 V are used on motorcycles. And batteries with a voltage of 24 V are operated on heavy trucks, special and military equipment.
To start the engine, its motoring is required, which is provided with the starter. And the starter is powered by a rechargeable battery. Therefore, they are often called starter batteries. At this point, the starter consumes much current (several hundred amperes), discharging the car battery. After the car started, the generator provides electricity production in the on-board network. The circuit is designed so that when traveling by car, the battery recharges and replenishes the charge given when the engine was started.
Battery’s principle of operation
The principle of operation of a lead-acid battery is based on the electrochemical reactions of Pb and PbO2 in the electrolyte. An aqueous solution of sulfuric acid is used as the electrolyte. Dozens of different reactions take place in the car battery, but we will consider only the main ones. When an external load is applied to the battery leads, the electrochemical process of interaction of the electrolyte with lead oxide starts. As a result of this reaction, metallic Pb is oxidized to PbSO4. When the battery is discharged, PbO2 is reduced at the anode, and Pb is oxidized at the cathode. The process of charging the car battery is reverse. When lead sulfate is consumed, the process of water electrolysis begins. During this process, hydrogen and oxygen are evolved at the cathode and anode, respectively. As a result of the evolving of hydrogen and oxygen, it seems that the electrolyte boils. It is better to avoid this process, because it consumes water, the electrolyte density increases, and because of the explosive mixture (hydrogen + oxygen) the danger of an explosion increases, too.
To maintain the required level of electrolyte, distilled water is added to the battery cells, if necessary.
Characteristics of car battery
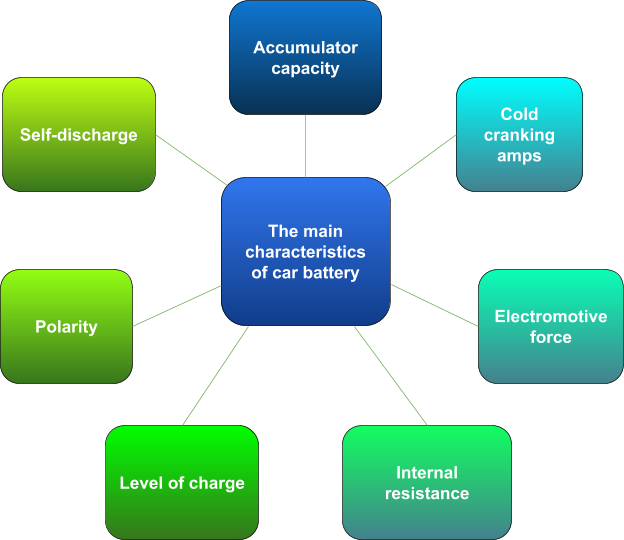
Accumulation capacity characterizes the amount of electricity given off when discharging to the minimum permissible voltage. The unit of capacity measurement is ampere-hours.
CCA (cold cranking amps) is also called the starting current. According to GOST, the declared starting current is checked after cooling the battery to -18 degrees Celsius. The car battery is discharged with a starting current in 30 seconds. After that, its voltage must be at least 8.4 volts. In the case of a discharge lasting 150 seconds, the voltage must be at least 6 volts.
The electromotive force of the battery (EMF) is a parameter that indicates the voltage at leads of the battery that is not loaded externally, and there are no leaks. EMF can be measured with a voltmeter or multimeter.
The internal resistance of the vehicle battery combines the resistance of separators, electrodes, electrolyte, leads and other battery cells.
The level of charge depends on many factors, and it’s difficult to measure the exact indicator. But the approximate level of charge is measured by EMF and electrolyte density.
Self-discharge of the battery is a process of the battery capacity’s reduction. It is caused by redox processes on electrodes.
In addition, it is important to consider the shelf life of the battery. The battery can be stored for some time in a dry-charged state before it reaches the consumer. It is also important to know the service life and design features of the battery (weight, size).
Types of car battery
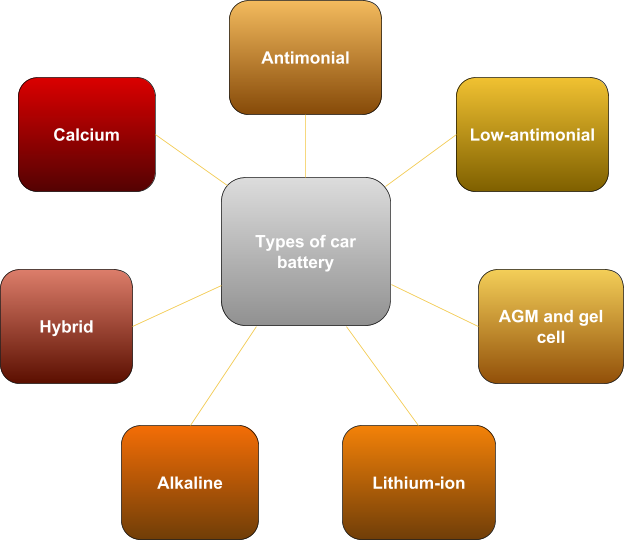
Antimonial batteries are gone and aren’t used for cars today. The electrodes of these batteries contain more than five percent antimony. Low-antimonial: low antimony plates were used to reduce decomposition of water into oxygen and hydrogen. But the problem of their service is still relevant. It is one of the most common types of batteries today. Lead grids became to be alloyed with calcium to solve the problem of water consumption and self-discharge reduction. At the same time, the problem of capacity loss during a deep discharge arose. Hybrid batteries are modern car batteries, which have become an attempt to find a compromise between low-antimonial and calcium batteries. AGM and gel batteries are relatively new car batteries. They became the next step in ensuring the safe operation of car batteries. Alkaline batteries – instead of acid, the role of electrolyte is played by alkali. The most common batteries are nickel-iron and nickel-cadmium. Lithium-ion batteries are quite promising, but today they aren’t widely used in cars due to a number of unsolved problems.

Even if you have no problems with the battery in your car, don’t forget to take an international driver’s license on the road, and if you still don’t have one, we invite you to quickly and easily process it on our website.






